MIGLYOL® as the Worldwide Benchmark for Injectables
29. June 2021
Injectables represent one of the most demanding segments in the scope of pharmaceutical dosage forms and are an integral part of the everyday life of many chronically ill people and in hospitals. The trend towards localised therapy paired with the development of smart, integrated delivery platforms meets the growing prevalence of diabetes, cancer, autoimmune diseases, etc. In addition to the established fields of application, it is predicted that the market for injectables will more than double by 2025 and the demand for raw materials for this top shelf segment will grow accordingly.
Today, injectables are part of everyday nursing and represent the parenteral administration of fluids for medical purposes, bypassing the digestive tract, Greek para: next to, enteron: intestine. If the active ingredient (API) has to be distributed quickly and evenly in the body, it is injected intravenously (directly into the vein) or intramuscularly (into the muscle). If you want to achieve slow absorption, subcutaneous (into the subcutaneous fat tissue) injection is used. Carrier media can be formulated as aqueous, lipid-based or emulsion-based, taking into account the solubility of the active ingredient, the intended route and absorption profile.
With the MIGLYOL® product range, IOI Oleo GmbH supplies pure auxiliary substances that make an important contribution to the production of consistent, stable, effective and ultimately safer medicines for the patient. Due to their polarity, our oils are perfect for poorly soluble APIs that are to be used for injectable formulations.
Repeated Rancimat tests prove that MIGYLOL® 840 and MIGLYOL® 812 N have a very high stability against thermal and oxidative stress, compared to other oils typically used in this field. Our products are based on high quality, plant-based raw materials from certified suppliers and are obtained at our GMP-certified production site in Witten in the heart of Europe, through solvent-free de novo synthesis. As we already reported in another article (EU GMP-Production – Quality Made in Germany you can rely on), IOI Oleo GmbH is one of the first manufacturers of GMP-certified auxiliary materials.
OXIDATION STABILITY WITH METROHM RANCIMAT TEST EQUIPMENT
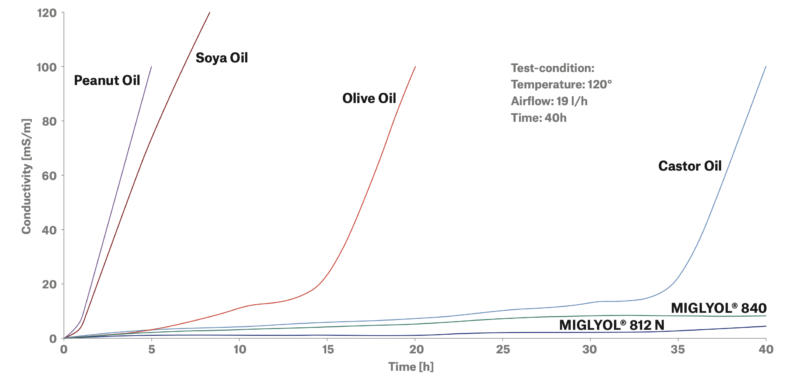
The conductivity increases exponential when the natural content of antioxidants in vegetable oil is consumed and the capicity to resist autoxidation is exhausted.
These properties, together with the detailed monitoring of our manufacturing and cleaning processes, result in products with a very high batch consistency and are therefore the ideal basis for pharmaceuticals with a long shelf life. With the MIGLYOL® product portfolio from IOI Oleo GmbH you have a number of additional benefits for injectables:
With our MIGLYOL® 812 N Drug Substance, we even have a product that is classified as an active ingredient and is used for parenteral nutrition. It consists of medium-chain triglycerides (MCT 60/40) and has already been a global benchmark for decades!
IOI Oleo GmbH is one of the leading manufacturers of lipid-based carriers for parenterals. If you would like to find out more about our products, take a look at our brochure (LINK) or get in touch with us!
Robert Radsziwill
Robert is the Business Development Manager for Functional Excipients in the Pharma Division of IOI Oleo GmbH. Prior to this role he held technical and commercial roles focusing raw materials used in the pharmaceutical and medical device industry for 13 years. In each position he leveraged his diploma degree in Business Chemistry earned at Westfälische Wilhelms-University to drive interdisciplinary projects and work as interface between commercial and technical decision makers. Understanding technical, commercial and regulatory drivers from ideation to successful project-completion is key for him. In his free time he enjoys guitar heavy music as well as cooking for and with family and friends.

