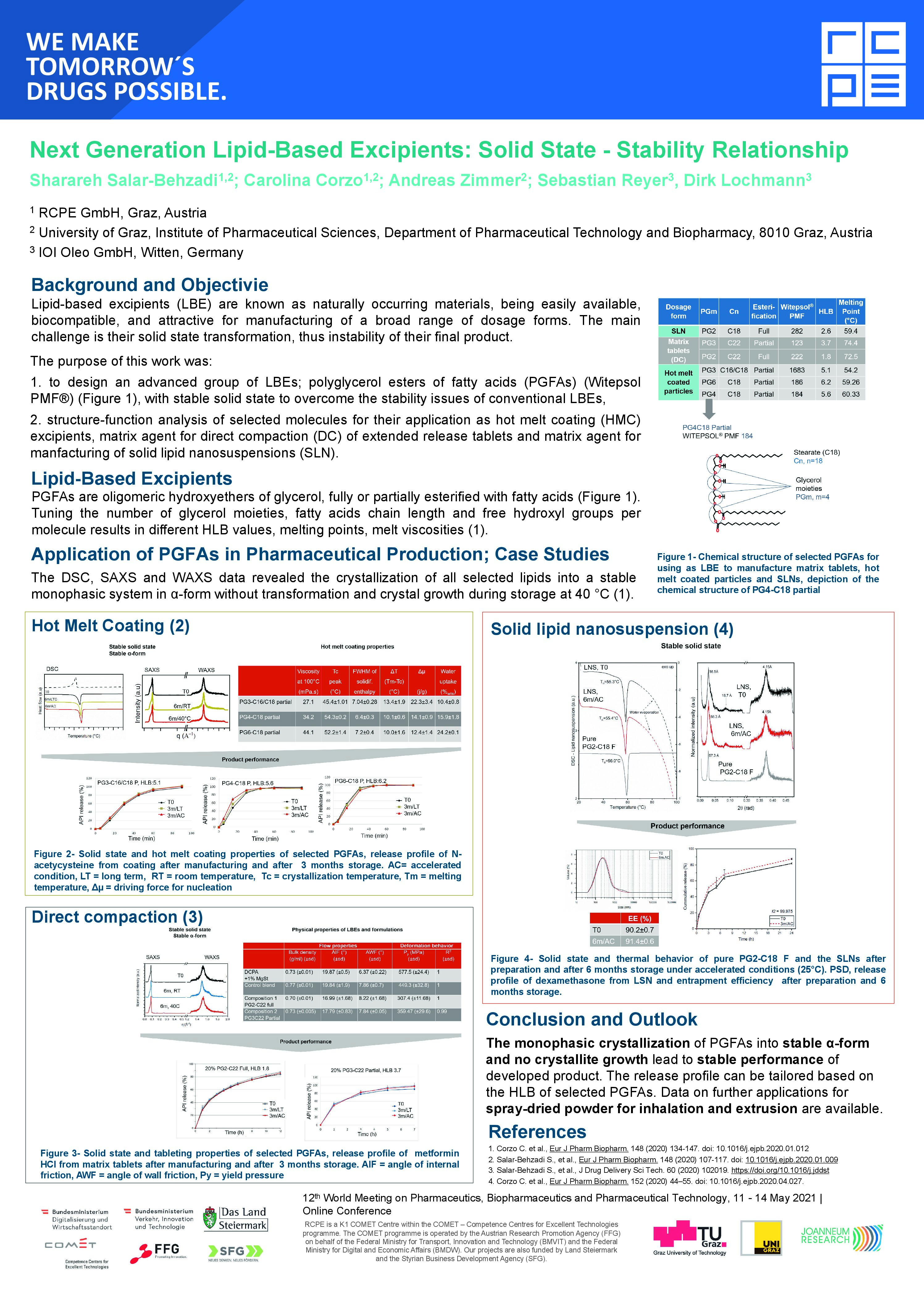
LipoGalen™
Our innovative polyglycerol esters now have a name – We’re thrilled to introduce LipoGalen™ as the brand name for our new family of Functional Lipid-Based Excipients.
In the last IOI newsletter, we announced the launch of our new family of functional, lipid-based excipients, the polyglycerol esters.
After intensive and dedicated research and development plus countless hours of brainstorming sessions, IOI Oleo GmbH finally unveils something truly special:
LipoGalen™ will be the name of our new brand, which will be making its debut at CPHI Frankfurt.
This launch marks a major milestone for us, and we couldn’t be prouder to share it with you. LipoGalen™ was born out of a deep understanding of the evolving challenges in pharmaceutical formulation. In contrast to common triglycerides, it crystallizes in one stable modification and does not show any polymorphism.
LipoGalen™ offers a versatile, forward-thinking platform designed to empower innovation across a wide range of applications:
- Rectal and vaginal dosage forms: A novel lipid base that offers new possibilities in the formulation development of such products, with advantages for patients.
- Controlled-release tablets: Matrix systems that allow precise control over drug-release profiles throughout the shelf-life, because of the superior properties of LipoGalen™ over traditional triglycerides.
- Solubilization and bioavailability enhancement: Perfect for hot melt extrusion, improving the performance of poorly soluble APIs, while offering the chance of working at reduced processing temperatures compared to the established polymers in this field of application.
- Taste masking: Effective via hot melt coating, helping improve patient compliance.
- Lubrication for tableting: Optimized for continuous manufacturing processes.
- Dermal formulations: Free from paraffins, supporting clean-label, skin-friendly solutions.
- Advanced delivery systems: Suitable for lipid nanoparticles and nano-emulsions, including inhalation therapies.
- 3D-printing: Compatible with melt extrusion techniques for personalized medicine and cutting-edge dosage forms.
We warmly invite you to join us at CPHI Frankfurt to discover how LipoGalen™ can elevate your formulation strategies and open up exciting new possibilities in drug delivery.
Let’s shape the future of pharmaceutical development — together.

A new generation of
lipid based excipients
What if a new generation of lipid-based excipients could lift advanced pharmaceutical drug development with poorly soluble APIs to its next level by providing a built-in fit-for-purpose feature for advanced processes such as hot melt extrusion, hot melt coating, particle engineering and 3D-printing?
More than 75% of new chemical entities are poorly soluble and require bioavailability-enhancing excipients. The application of excipients in advanced pharmaceutical processes, aligned with advanced approaches towards personalized medicine, requires special features. Lipid-based excipients (LBEs) are „natural contenders“ for the tasks. However, until now, it has not been possible to fully exploit the potential of LBEs. The main challenge is the unstable solid state of current LBEs, e.g. polymorphism, leading to instability of the final dosage form. Compared to polymers, the low melting temperature of liquids, combined with highly crystalline arrangement, limits their application in advanced pharmaceutical processes.
Reason why

Full Commitment to
Sustainability
90% vegetable renewable raw materials
- RSPO SCCS certification
- associate member of the PSCI
- ISO 14001 certified
- EMAS conform

Highest Quality & Compliance Standards
100 years of expertise in lipids Made in Germany
- EU GMP certified
- US FDA cGMP inspected
- reliable batch to batch consistency

Innovation driven
Expert
Leading global expert and innovator of functionalised ester-based lipids with added value for pharma solutions
How this new generation of LBEs could serve your fit for purpose needs is described in the publications and posters listed below.
Novel approach for overcoming the stability challenges of lipid-based excipients
Part 1: Screening of solid-state and physical properties of polyglycerol esters of fatty acids as advanced pharmaceutical excipients
Carolina Corzo a,b, Diogo Gomes Lopes a, Dirk Lochmann c, Sebastian Reyer c, Michael Stehr c, Sharareh Salar-Behzadi a,b
Novel approach for overcoming the stability challenges of lipid-based excipients
Part 2: Application of polyglycerol esters of fatty acids as hot melt coating excipients
Sharareh Salar-Behzadi a, Carolina Corzo a, Diogo Gomes Lopes a, Claudia Meindl c, Dirk Lochmann d, Sebastian Reyer d
Novel approach for overcoming the stability challenges of lipid-based excipients
Part 3: Application of polyglycerol esters of fatty acids for the next generation of solid lipid nanoparticles
Carolina Corzo a,b, Claudia Meindl c, Dirk Lochmann d, Sebastian Reyer d, Sharareh Salar-Behzadi a,b
Eleonore Fröhlich a,b, Kristin Öhlinger b, Claudia Meindl b, Carolina Corzo c, Dirk Lochmann d, Sebastian Reyer d, Sharareh Salar-Behzadi a,c
Carolina Corzo a,b, Diogo Gomes Lopes a, Dirk Lochmann c, Sebastian Reyer c, Michael Stehr c, Sharareh Salar-Behzadi a,b
Sharareh Salar-Behzadi a, Carolina Corzo a, Diogo Gomes Lopes a, Claudia Meindl c, Dirk Lochmann d, Sebastian Reyer d
Carolina Corzo a,b, Claudia Meindl c, Dirk Lochmann d, Sebastian Reyer d, Sharareh Salar-Behzadi a,b
Eleonore Fröhlich a,b, Kristin Öhlinger b, Claudia Meindl b, Carolina Corzo c, Dirk Lochmann d, Sebastian Reyer d, Sharareh Salar-Behzadi a,c
If you have further specific needs or questions or in case you have already been confronted with the unstable solid state of existing LBEs in your formulation, we are happy to support you to overcome the challenge of polymorphism.
Please don´t hesitate to contact us.