Polyglycerol esters – the new generation of functional lipids for pharmaceutical applications
18. September 2025
When a challenge becomes an opportunity
In the world of pharmaceutical development, there is a constantly growing hurdle: more and more innovative active ingredients are poorly soluble in water or have a low permeability – and are therefore difficult for the human body to absorb. The result? Active ingredients with high therapeutic potential cannot achieve their therapeutic goal. But this is precisely where the newly developed group of excipients, the innovative lipid-based polyglycerol esters, offers new possibilities in the development of formulations.
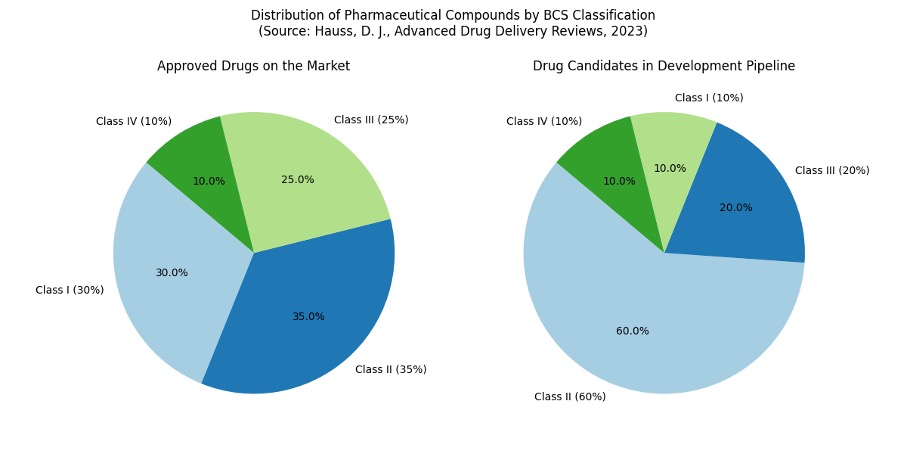
The available data speaks for itself: 35% of drugs approved today belong to BCS class II, which means they are poorly soluble in aqueous environments but have good permeability. In the development pipeline, this figure is even more dramatic: 60–70% of all drug candidates have poor solubility.
In recent decades, this limitation has prevented many drugs with promising therapeutic potential from being made available to patients in the form of a medicine.
Why lipids?
Functional lipids offer the opportunity to unlock this potential. Polyglycerol esters are amphiphilic lipids which, due to their nature, have a lipophilic molecular component but also have the ability to absorb significantly larger amounts of water than classic triglycerides. In a broader sense, they can be described as hydrophilic lipids.
This special group of excipients has the potential to redefine the rules of the game. Functional lipids are not only carrier substances, but they are also key components in the development of stable, effective, and well-tolerated drug forms that deliver such APIs safely to their site of action.
The polyglycerol ester group of excipients – versatile, stable, well-tolerated
IOI polyglycerol esters are the result of targeted development for pharmaceutical applications. The substances are characterized by the following properties:
Discover our pharmaceutical lipids!
Innovation begins with the right partner. Let us work together to develop next-generation drug forms. Contact our team of experts for individual consultation and formulation support (You can find our team here).
Or visit us directly at CPHI and speak to our team.
Dr. Thomas Rillmann
Dr. Thomas Rillmann studied Chemistry at the Technical University of Munich and Pharmacy at the Technical University of Braunschweig, finishing his Ph.D. thesis under the supervision of Prof. Dr. Dr. h.c. Claus Führer. He has more than 25 years of experience in the pharmaceutical industry, mainly working in product development and transfer. He started his professional career at Hexal Pharmaforschung GmbH working on multi-particulate controlled release dosage forms, then joining the STADA group, building up the Pharmaceutical Development Department from 2003 to 2010 and the Product Transfer Department from 2008 to 2009. After a 4-year’s stint in the pharma business segment of BASF SE, he held to two positions in pharmaceutical manufacturing, the last position as a Site Head for Riemser Pharma GmbH. Thomas Rillmann joined IOI Oleo GmbH on January 1st 2023 as Head of Business Development Functional Excipients.